
Infrastructure Development Projects
Infrastructure Development Projects support ECRIN’s core mission by strengthening its capacity, enabling ECRIN to be on the leading edge in clinical research, enhancing its visibility and developing synergies with the European research infrastructure community. Through the projects, ECRIN contributes to creating tools and methodologies, setting up partnerships with medical specialties and providing resources and services to foster clinical research in Europe as well as in collaboration with international partners.
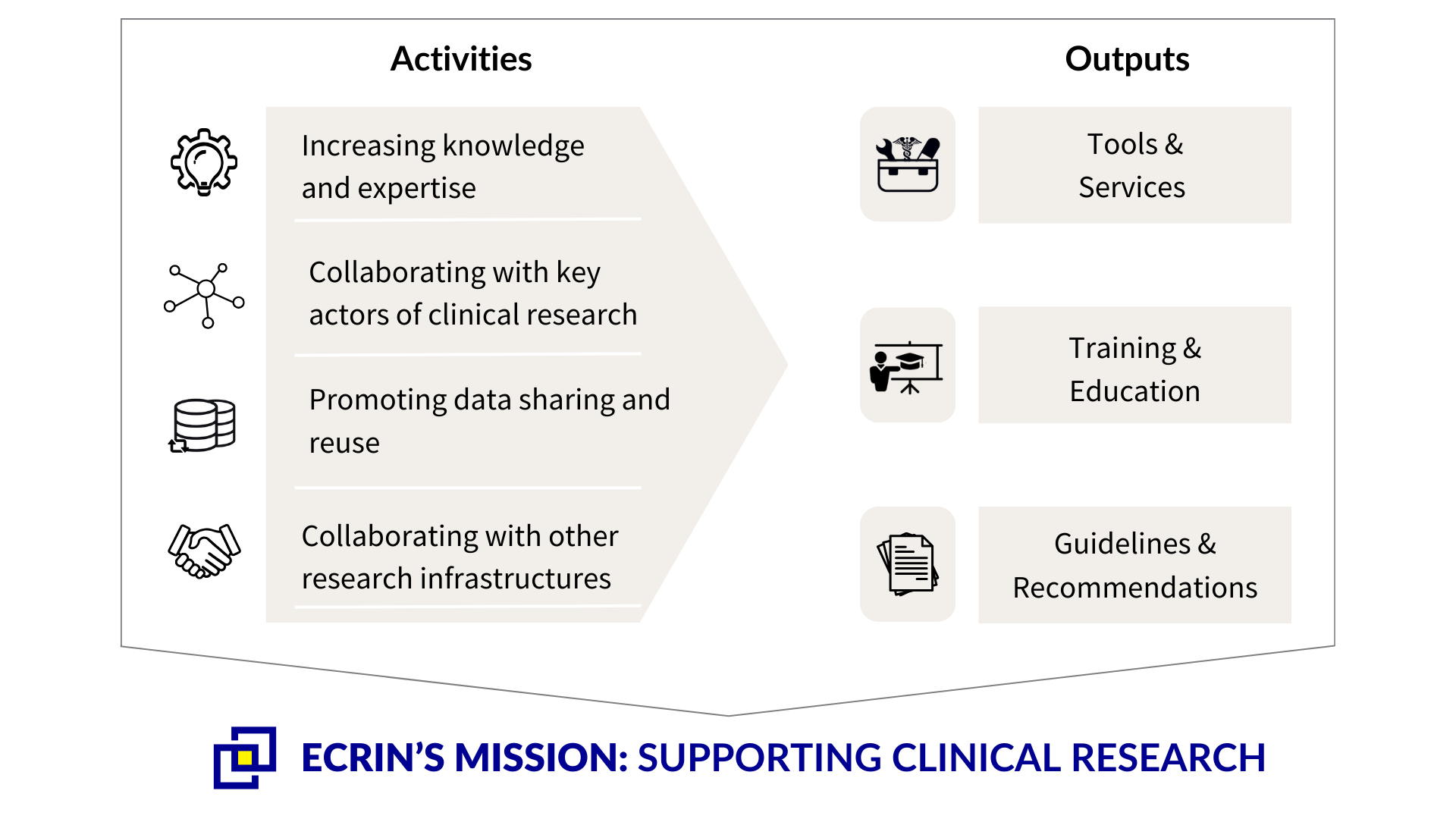
These infrastructure projects are diverse and multidisciplinary in nature. They cover a wide breadth of areas and lead to concrete outputs (as seen above). In the accompanying tabs, we go into more detail on some of the outputs of the work carried out by the infrastructure development projects. Many more of the tools, policies, training and outcomes are centralised on this website.
![]() Discover the ECRIN projects portfolio.
Discover the ECRIN projects portfolio.
Within the context of the infrastructure development projects, different tools are developed to further advance knowledge, facilitate finding relevant clinical research information and data, and create links between researchers.
Support to specific user communities
The Paediatric tools, developed through the ECRIN coordinated PedCRIN project, support the set-up and management of neonatal and paediatric multinational clinical trials across Europe.
The Rare Disease Clinical Trial Toolbox, developed at ECRIN through EJP-RD, centralises resources in support of clinical trials conducted for the rare diseases community but can also be of use to a broader community.
Data access and reuse
The Clinical Research MetaData Repository is the online tool, created in XDC and further developed in EOSC-Life, to help scientific researchers find documents and data linked to a clinical research study, and to obtain information on the accessibility of those data.
New trial design
The Adaptive Platform Trial Toolbox developed in the RECOVER and EU-RESPONSE projects centralises resources on this new clinical trial design in relation to design, regulatory issues, conduct, statistics and data management, reporting and feedback from existing trials.
Many other tools exist, find the one that matches your needs today.
![]() Discover the ECRIN tools page.
Discover the ECRIN tools page.
While all projects integrate communication and dissemination, certain projects have communications and / or training as their primary outcome.
Networking and sharing
The RI-VIS project aimed to increase the visibility of European research infrastructures (RIs) to new communities in Europe and beyond. The PerMed projects EULACPerMed & EU-AfricaPerMed, look to bring together research communities in other corners of the world to network and build.
Development of training to meet the clinical research community needs
Innovative and just-in-time training can be developed for various user groups through the projects, in order to facilitate the uptake of project findings. Currently, multiple levels of online learning are made available from the recommendations of the PERMIT project. Training is also made available to support user groups within a consortium as is the case with the VACCELERATE project where ECRIN shares its expertise in multinational clinical trials.
Informing the general public
The EuroGCT project looks to share accurate information with the public and researchers on gene and cell therapy.
A very different example of the available training material comes from the ECRAN project: a video series in all European languages that describes what a clinical trial is and what it looks to do.
Development of recommendations
Projects can also assemble field experts to drive the development of current standards for the uptake by different stakeholder groups to improve clinical research across Europe. The ECRIN coordinated PERMIT project focussed on developing methodological recommendations across the personalised medicine pipeline to support all the different stakeholders and the public in improving reproducibility, transparency, harmonisation and quality research in general.
Supporting the RI community through policy development
ECRIN contributed, through the ERIC-Forum project, to the development of three different policy reports that underscore the added value of the ERIC system as the backbone of the ERA and their needs to further contribute to a knowledge-based resilient European research ecosystem.
The RI VIS project developped three White Papers in collaboration with national and international stakeholders to better inform international collaborations across three world regions: Africa, Latin America and Australia


