
Data Centre Certification
ECRIN's Data Centre Certification Programme
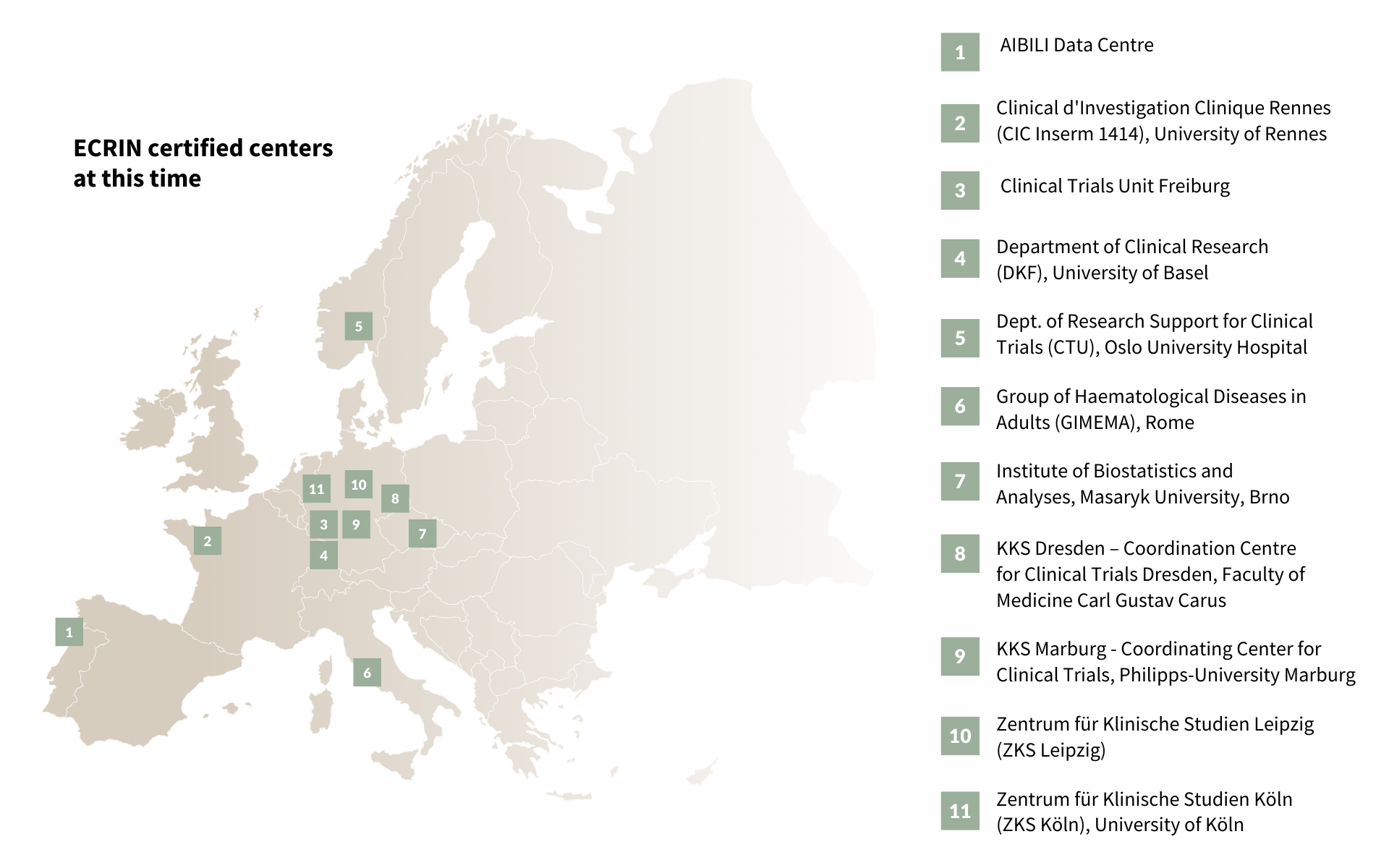
 ECRIN’s Data Centre Certification programme is ISO 9001:2015 certified and was developed to audit European, non-commercial data centres using ECRIN IT/DM standards, to confirm their ability to provide compliant, effective, and efficient data management services for controlled clinical trials. The goal is to enhance high-quality data management services in non-commercial clinical trials and to contribute to the harmonisation of European practice in data management. Since it was set up in 2014, 19 European data centres have been certified. Those currently certified or in the process of recertification can be seen in the image below.
ECRIN’s Data Centre Certification programme is ISO 9001:2015 certified and was developed to audit European, non-commercial data centres using ECRIN IT/DM standards, to confirm their ability to provide compliant, effective, and efficient data management services for controlled clinical trials. The goal is to enhance high-quality data management services in non-commercial clinical trials and to contribute to the harmonisation of European practice in data management. Since it was set up in 2014, 19 European data centres have been certified. Those currently certified or in the process of recertification can be seen in the image below.
ECRIN’s certification programme assesses the units for compliance with published ECRIN data standards, performing an audit of the unit’s data management activities and of the IT infrastructure used to support those activities [1]*.
*See references section
2025 Call conditions & application process
Conditions/ Eligibility of data centres:
- The applicant clinical data management centre is part of (or affiliated to) the national network of an ECRIN Member (Czech Republic, France, Germany, Greece, Hungary, Ireland, Italy, Norway, Poland, Portugal, Slovakia, Spain and Switzerland).
- The applicant data centre has been preselected and approved by the coordination of the national network.
- The applicant clinical data management centre is ready for audit within 6 months following the evaluation of the “Initial Application Questionnaire”.
- No charges are applied to applicants from Member countries. Data centres from Observer countries will be asked to reimburse the certification costs (direct expense costs, approximately 8.500€).
Application process:
- Applicants send a request to ECRIN (DCC.contact@ecrin.org) to receive a link to an automated questionnaire, "Initial Application Questionnaire"
- Applicants must submit their completed questionnaire by 22 September 2025, 17h CEST.
- Completed questionnaires will be evaluated by the Independent Certification Board Secretariat to confirm the readiness for audit. The main criterion is the ability of the data centre to meet the IT/DM standards demonstrated through at least 2 recent studies (data centre being responsible for the data management).
- Successful applicants will then move to audit planning.
All certification decisions are taken by the Independent Certification Board, based on the results of the audit of the data centre’s system and procedures.
Initial audits are preferably performed on-site, however, the decision to conduct on-site or remote audit is based on the auditors ‘decision.
The audit team is composed of up to three ECRIN auditors qualified in IT/DM clinical trial quality.
Audits are conducted in English and involve when possible one person fluent in the national language and able to read documents in the national language.
INITIAL CERTIFICATION
Pre-requisite: the data centre must be selected or receive the agreement from the national network.
Audit Process : scheme
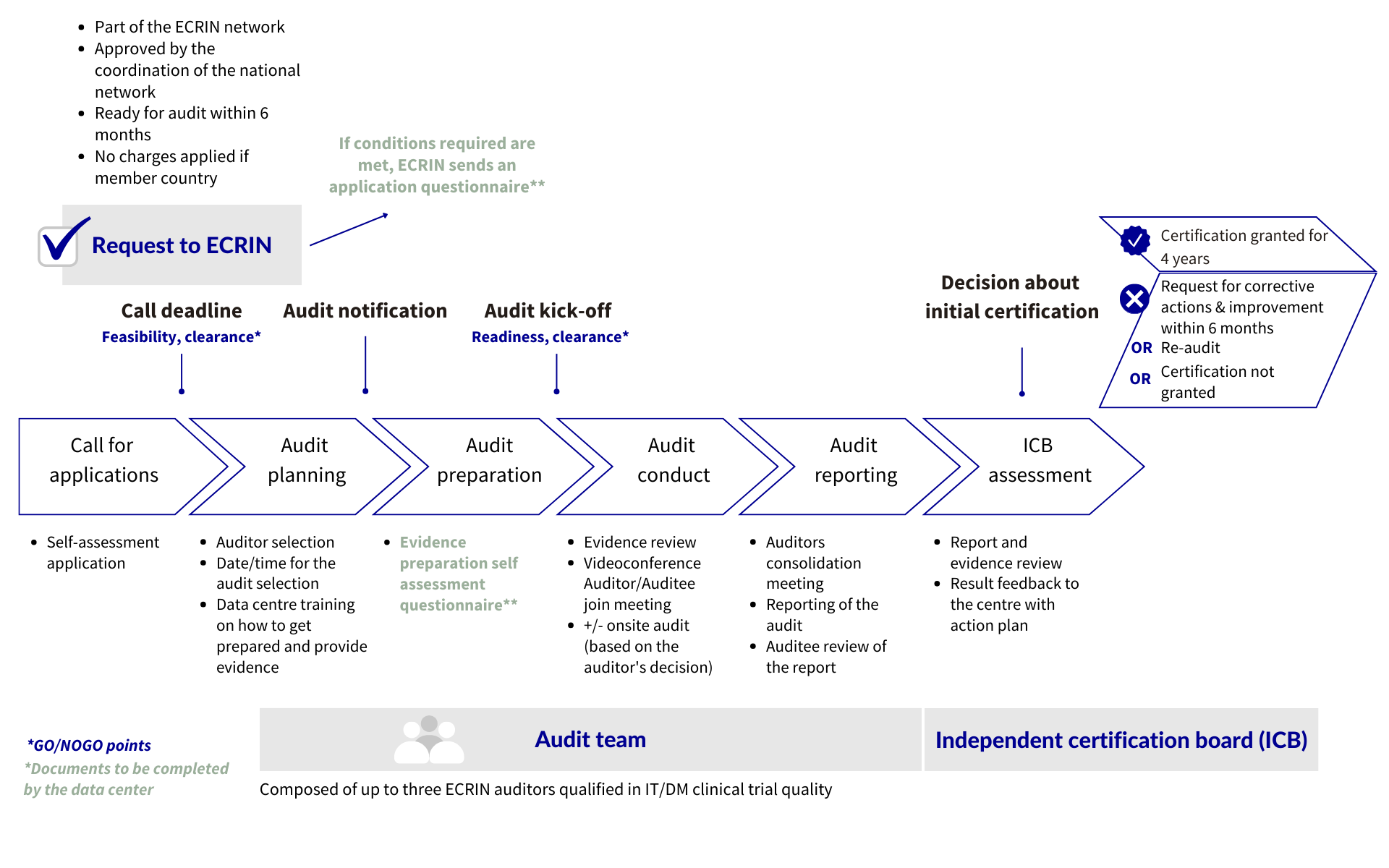
Decision: the decision of the Independent Certification Board will be one of the following:
- Certification granted for 4 years (when all standards are met)
- Request for Corrective Actions or Improvements within 6 months to evidence compliance with standards that were not met. Certification decision then reconsidered.
- Re-audit: re-audit within agreed deadline to evidence compliance with standards not met. Certification decision then reconsidered.
- Not granted: if level of non-compliance is such that corrective actions and re-audits are not realistic options.
CERTIFICATION RENEWAL
An alert letter will be sent by ECRIN Independent Certification Board secretariat 6 months before certification lapse.
The data centre should, before the certificate lapses, send an official request for certification and fill in the online renewal application.
In the absence of a request within 6 months following certificate lapse, the data centre is withdrawn from the list of certified data centres.
Audit process: scheme
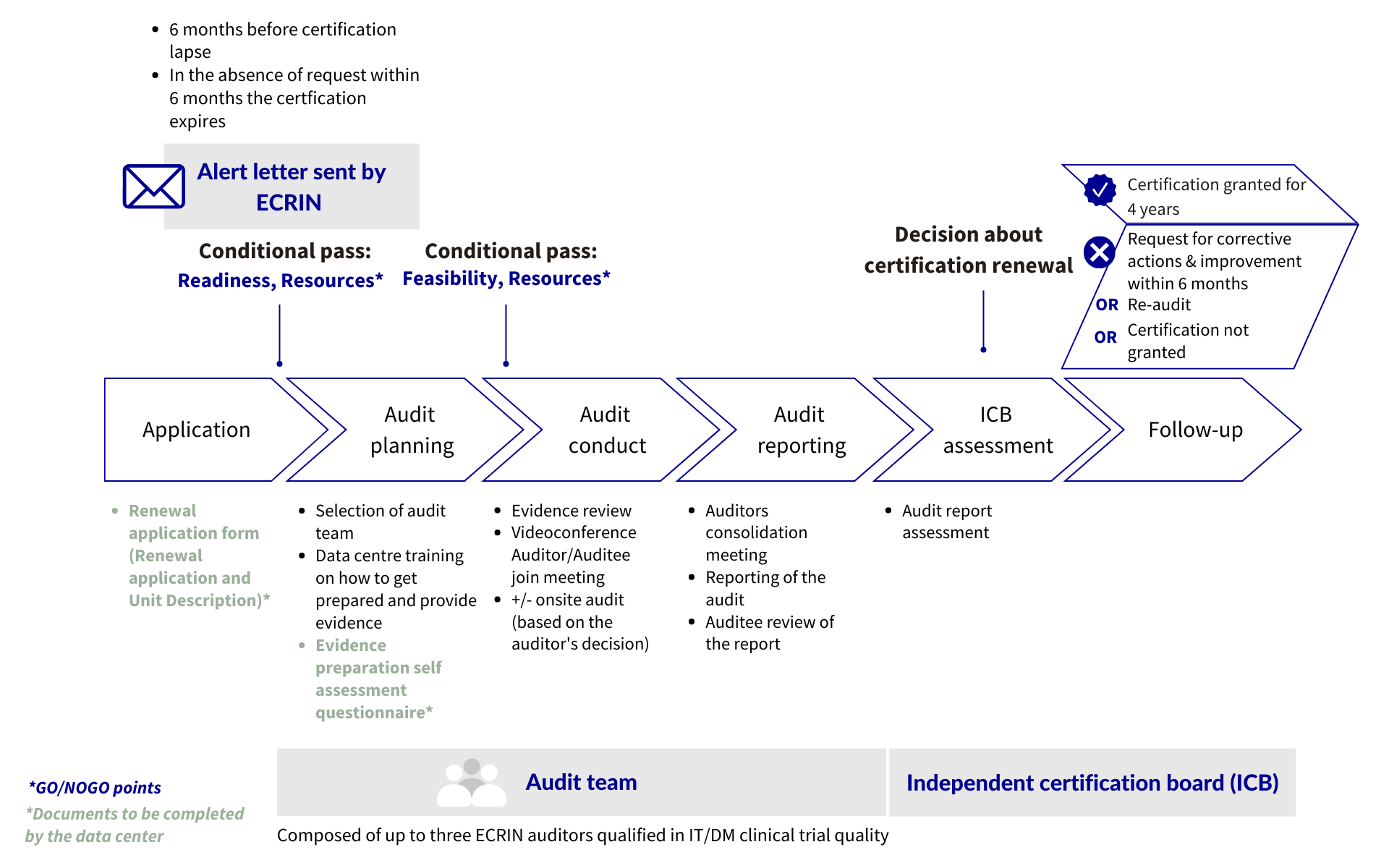
Decision: the decision of the Independent Certification Board will be one of the following:
- Certification granted for 4 years
- Request for Corrective Actions or Improvements within 6 months to evidence compliance with standards that were not met. Certification decision then reconsidered.
- Re-audit: re-audit after within agreed deadline to evidence compliance with standards not met. Certification decision then reconsidered.
- Not granted: if level of non-compliance is such that corrective actions and re-audits are not realistic options.
In 2018, it was decided to expand the programme as a pilot beyond Europe. Non-EU interested countries could apply for one pilot audit and request the training of local auditors. Three auditors were trained and two data management centre were certified in Japan.
In the continuation of the pilot initiated in Japan to globalise the Data Centre Certification programme, ECRIN conducted an initial audit of the Medical Research Collaboration Center (MRCC) at Seoul National University Hospital in Seoul, South Korea, from January 13th to 17th, 2020. The Data Centre Certification was awarded to MRCC at the end of 2020.
 The current criteria for certification with explanation and elaboration (version 5.0 from June 2023) are now available:
The current criteria for certification with explanation and elaboration (version 5.0 from June 2023) are now available:
ECRIN certification standards
The first version of the ECRIN certification standards was created in 2010 and published in the journal Trials in 2011 [3]. Updates of the standards (Version 2.2) were published in 2013 in Trials [4], in 2015 (Version 3.0) [5] and in 2018 (Version 4.0) in the EU database Zenodo [6].
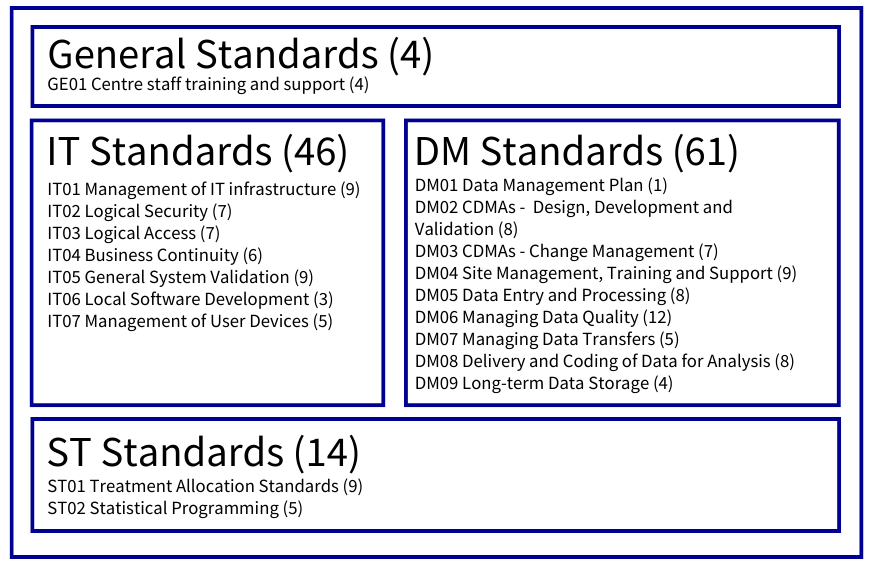
The standards are split into three main areas, and one optional area as shown in the picture. They provide a clear interpretation of regulatory and good practice requirements, and act as a guide to establishing and managing high-quality data management services.
Publications & Reports
1. Ohmann et al.: Raising standards in clinical research–The impact of the ECRIN data centre certification programme, 2011–2016. Contemporary Clinical Trials Communications, 2017; 153:159. DOI: 10.1016/j.conctc.2017.02.005 (https://www.sciencedirect.com/science/article/pii/S2451865416300825)
2. Crocombe et al.: Requirements for Certification of ECRIN Data Centres, with Explanation and Elaboration of Standards, Version 5.0. 28 June 2023.Zenodo. https://doi.org/10.5281/zenodo.8090816 (https://zenodo.org/record/8090816)
3. Ohmann et al.: Standard requirements for GCP-compliant data management in multinational clinical trials. Trials, 2011; 12:85. DOI: 10.1186/1745-6215-12-85 (https://trialsjournal.biomedcentral.com/articles/10.1186/1745-6215-12-85)
4. Ohmann et al.: Revising the ECRIN standard requirements for information technology and data management in clinical trials. Trials, 2013; 14:97. DOI: 10.1186/1745-6215-14-97 (https://trialsjournal.biomedcentral.com/articles/10.1186/1745-6215-14-97)
5. Canham et al.: Requirements for Certification of ECRIN Data Centres, with Explanation and Elaboration of Standards, Version 3.0. Zenodo, 2015. DOI: 10.5281/zenodo.32690 (https://zenodo.org/record/32690#.XL8WqTAzbct)
6. Canham et al.: Requirements for Certification of ECRIN Data Centres, with Explanation and Elaboration of Standards, Version 4.0. 27 April 2018. DOI: 10.5281/zenodo.1240941 (https://zenodo.org/record/1240941#.XL8VATAzbct)
REPORT: "Survey on impact of ECRIN data centre certification on inspections by regulatory authorities" (C. Ohmann, C. Toneatti, August 2019)
Certification Programme Training and Presentations:
ECRIN – CDISC joint training to partner CTUs and auditors, April 11th, 2019
EU Quality Conference, Dublin, November 6-8th 2019, Title: Enhancing High-Quality Data Management Services in European Non-Commercial Clinical Trials
23rd DIA Japan Annual Workshop for Clinical Data Management, February 5-6, 2020, Tokyo, Title: Activity report of CRIGH Project 6: Clinical Data Management
DIA Europe Annual Workshop Abstract Virtual, July 2020, Title: Enhancing High-Quality Data Management Services in Non-Commercial Clinical Trials.


