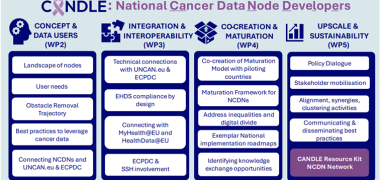
New publication - Medical journal requirements for clinical trial data sharing: Ripe for improvement
ECRIN contributed to a policy forum “Medical journal requirements for clinical trial data sharing: Ripe for improvement” was published 25 October 2021 in PLOS Medical.
Efficient sharing and reuse of data from clinical trials are critical in advancing medical knowledge and developing improved treatments. In 2016 the International Committee of Medical Journal Editors (ICMJE) wrote that responsible sharing of de-identified clinical trial data was an "ethical imperative". ICMJE proposed that same year to make data sharing mandatory, one year later it stepped back, and only required a data sharing statement (could be yes or no) in any published article and in study registration (e.g. on clinicaltrials).
The authors believe that the ICMJE clinical trial data sharing policy is inadequate at present. Although data sharing plans help increase transparency, they do not ensure that data are shared and they are often inadequately implemented.
For funders with a data-sharing policy, the implementation of the policy in study registration was limited for commercial funders and of concern for non-commercial funders. Moreover, intent to share data is not correlated with data re-use or effective data sharing.
It is suggested that the ICMJE should adapt a stronger policy on data sharing that is enforced rigorously in all ICMJE members and affiliated journals. The policy should include a strong evaluation component to ensure that all clinical trial data are shared, their value maximized, and data producers incentivized.
The full article is available here