Metadata Repository available for all clinical research
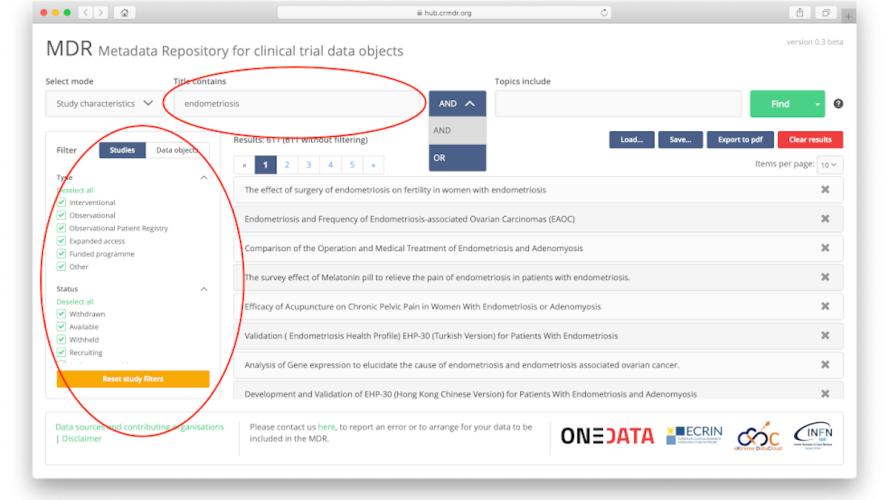
The Clinical Research Metadata Repository is the online tool to help scientific researchers find documents and data linked to a clinical research study, and to obtain information on the accessibility of those results. ECRIN made the Clinical Research Metadata Repository freely available for all scientific researchers on (nearly) all diseases, cures & vaccines.
The CRMDR.org is an easy to use platform for all clinical research, please find more information on our special page: https://ecrin.org/tools/clinical-research-metadata-repository