
Training: Everything you need to know about submitting a European Multinational Clinical Study Proposal

Through this training attendees will learn about the different sections of the Horizon Europe application form. The webinars will delve into different elements to consider for the submission process. To consolidate the online session 4 parallel onsite sessions will be held in ECRIN Member countries.
This training is open to Investigators and project managers in ECRIN Member and Observer countries considering applying for funding for clinical study projects through the Horizon Europe programme.
Individuals can either register for any of the webinars (from a single webinar through to the whole series) or they can apply to attend all webinars and the onsite training (planned pending sufficient interest in Jan/Feb 2024 in Brno, Lisbon, Paris, Warsaw). The applications for the full training can be submitted for an individual or a team (investigator & project manager).
Applications for the full training are now closed. The coordination team will reach out to those accepted for the full training programme shortly thereafter.
The training is available at no cost to the participants. For those applying for the full training, including the webinar series and the 1 day onsite training, they will be responsible for covering the cost of their travel and accommodations to the onsite training.
Below we will delve into the planned training series and the application process.
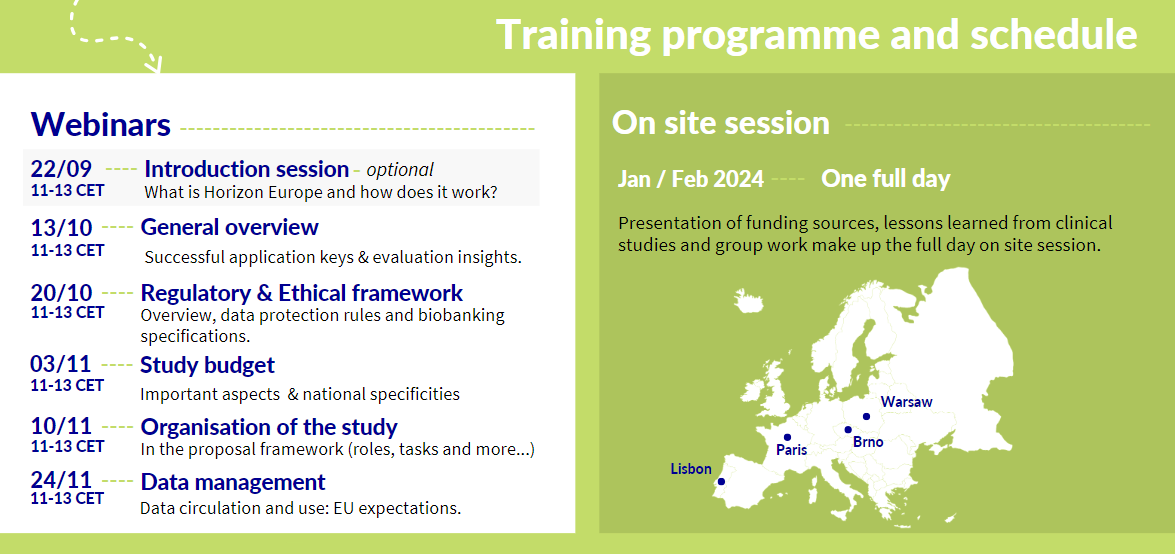
More information on the webinar series
All webinars are hosted on Fridays between 11-13CET
Introduction session
22 September
This session focuses on what is Horizon Europe, how it works and where one can find key information on upcoming calls, relevant timelines and local resources. It will be hosted by the Czech and Portuguese National Contact Points. It has been developed for those with little to no knowledge and or experience in applying to the Horizon Europe (or H2020) programmes. This is considered an optional session (for the onsite training).
The remainder of the webinars are compulsory for any person who will attend the onsite training.
General Overview
13 October
In this webinar, we will focus on how to develop a successful application. The organisation of the proposal application will be presented (excellence, impact, quality and efficiency of the implementation). Moreover, suggestions will be provided for robust proposal development including key steps, and support from specific organisations.
We will then focus on the aspects that are specific to clinical studies and close with an overview of how proposals are evaluated.
Regulatory and ethical framework
20 October
In this webinar, we will focus on the European and National regulatory requirements, ethical requirements and general data protection regulations. We will also look at elements relating to biobanking.
Study Budget
3 November
In this session, we will focus on EU format for the budget. The focus will also cover important aspects when preparing a budget and considerations for national specificities.
Organisation of the Study
10 November
In this webinar, we will describe how one should organise the clinical study within the proposal framework including elements such as roles and responsibilities, task distribution, study coordination, risk assessment, study management and close out.
Data management
24 November
This last webinar, will look at the aspects that are generic to any project and those that are specific to a clinical study. Elements related to data circulation and use will also be covered, as well as, the EU expectations on questions related to data management.
More information on the Onsite training
Upon registration please indicate the location(s) of interest to you.
12 January 2024 | Lisbon, Portugal
25 January 2024 | Brno, Czech Republic
7 February 2024 | Warsaw, Poland
8 February 2024 | Paris, France
Please note that you are responsible for your own travel and accommodation. As places are limited please only apply if you are sure to be able to attend the session. Application does not guarantee a spot, if necessary due to a high number of applications selection will be made based on the information included in the registration form. A waitlist will be created in the event of large number of candidates and spots will be given if attendees do not fulfil the necessary requirements to attend all webinars or have a conflict with the date of the onsite training.
Throughout the course of the day, attendees will have the opportunity to analyse different aspects of the proposal submission procedure. In the morning, we will go through two different use cases. Where possible, the use case will highlight elements of the proposal application process for projects where the host country has had a key role. You will have the opportunity to discuss with some of those involved in the process. In the afternoon attendees will work together in groups to prepare a proposal (or elements of a proposal) for submission.
Please note that it is compulsory for all attendees of this one day onsite training to join us for the webinars.
Registration
To register you must be situated in an ECRIN Member or Observer country (Czech Republic, France, Germany, Greece, Hungary, Ireland, Italy, Norway, Poland, Portugal, Slovakia, Spain, Switzerland). You can register directly for the webinars and select those that are of interest to you or you can apply for the full training including the one day onsite training. For those that applied for the onsite training you will be automatically registered for the relevant webinars.
For any questions or for more information contact training@ecrin.org.
For more information on future iterations and other training initiatives offered by ECRIN please visit our training page



