CANDLE
National CAncer data Node DeveLopErsFunding programme: Horizon Europe (N 101214368)
Budget: €3M
Coordinator: Health-RI
Duration: 3 years (2025 - 2028)
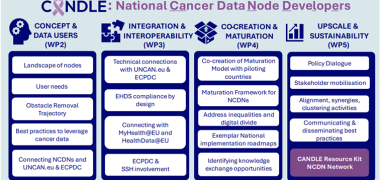
CANDLE will play a strategic role in supporting the European Health Data Space (EHDS) by establishing a federated network of national nodes in the cancer domain. These nodes can be seen as demonstrators for national disease-specific implementation of the EHDS, aligning EU-wide health data policies with regional and national requirements. Furthermore, CANDLE puts into practice the EU Mission on Cancer and Europe’s Beating Cancer Plan by supporting their implementation on national level and fostering dialogue with other major initiatives.
The CANDLE Consortium includes partners from Member State governance bodies, cancer care, patients and public health, as well as six major European Research Infrastructures. Thus, the consortium is well-suited to ensure the adoption of best practices in data sharing, enhance maturity in data access, and foster cross-domain collaboration
The National Cancer Data Nodes implemented in CANDLE will closely link to other European cancer data initiatives: 1) the UNCAN.eu platform, a federated cancer data infrastructure being developed under the upcoming EU project UNCAN-CONNECT, and 2) the European Cancer Patient Digital Centre (ECPDC).
ECRIN's role in CANDLE
In CANDLE, ECRIN leads the effort to develop and share clear, practical guidelines for preparing and reusing cancer-related data. This involves liaising with a number of European Research Infrastructures, initiatives, prospective national cancer data nodes (NCDN) and key players from the upcoming EHDS. The objective is to identify current needs, challenges, and best practices in areas such as data management, interoperability, quality, provenance, and semantic integration. This will facilitate the identification of effective practices, and the management of cancer data is of a high quality, consistent, and beneficial to research, while also protecting patient privacy and data security. ECRIN is also contributing to aligning these efforts with the broader European Health Data Space. It brings its expertise in clinical trial data to help ensure that national cancer data nodes are secure, standardised, and ready to share high quality data responsibly. Finally, ECRIN supports the development and testing of a new "CANDLE Maturation Model." This tool will help NCDNs assess their current practices and identify areas for improvement.