
Training: 2nd Edition - Everything you need to know about submitting a European Multinational Clinical Study Proposal
After the success of the first edition, we have decided to open a second edition of our EYNTK about submitting an EU multinational clinical study proposal training.
Through this training, attendees will learn about the different sections of the Horizon Europe application form. The webinars will delve into different elements to consider for the submission process. To consolidate the online session, 4 parallel onsite sessions will be held in ECRIN Member countries.
The online portion of the training is open to Investigators and project managers in Horizon Europe eligible countries, considering applying for funding for clinical study projects through the Horizon Europe programme.
Individuals can register for any of the webinars (from a single webinar through to the whole series). Access to the complementary onsite trainings will be opened at a later date (these are only open to individuals in ECRIN member countries). The eligibility requirements of the onsite trainings will include participation in the earlier webinars. The onsite sessions are planned for January/February 2026 in Madrid, Rome, Paris, and Warsaw.
The training is available at no cost to the participants. For those applying for the full training, including the webinar series and the 1 day onsite training, they will be responsible for covering the cost of their travel and accommodations to the onsite training.
Below we will delve into the planned training series and the application process.
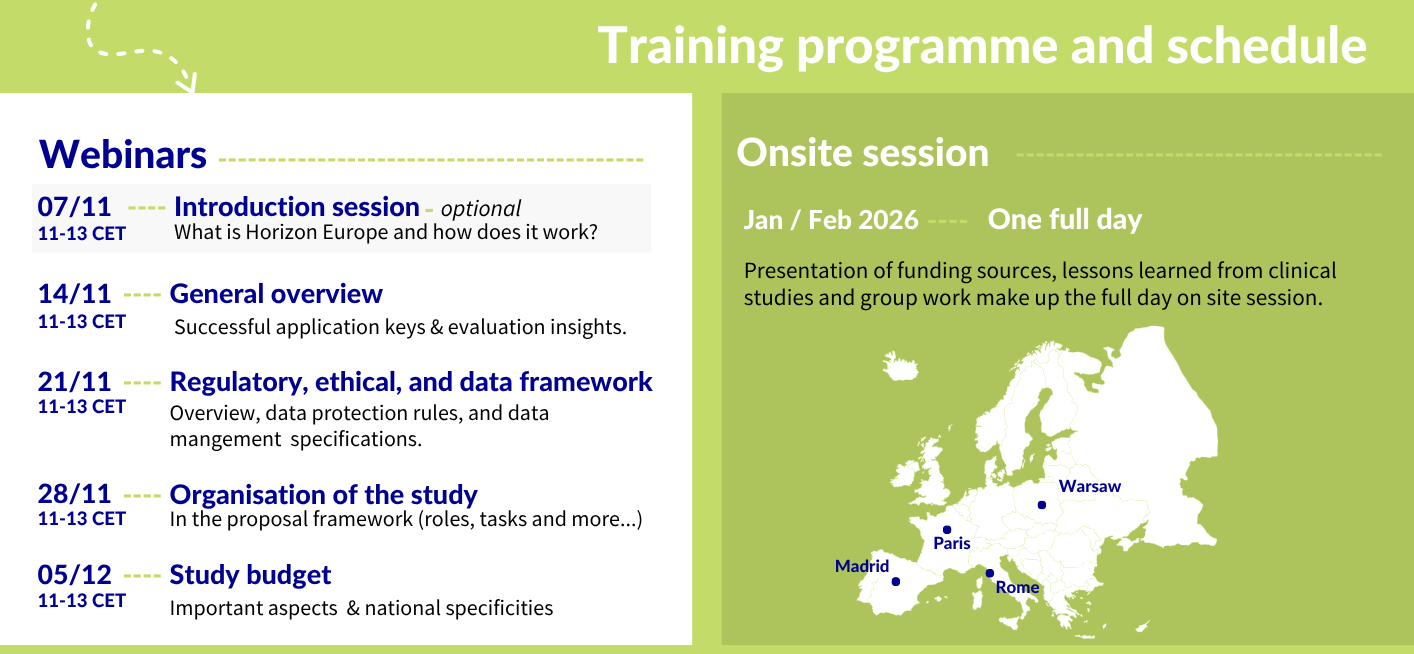
More information on the webinar series
All webinars are hosted on Fridays between 11-13CET.
Introduction session
Friday, 7 November 2025 11-13 CET
This session focuses on what is Horizon Europe, how it works and where one can find key information on upcoming calls, relevant timelines and local resources. It will be hosted by the Swiss and Portuguese National Contact Points. It has been developed for those with little to no knowledge and or experience in applying to the Horizon Europe (or H2020) programmes. This is considered an optional session (for the onsite training).
The remainder of the webinars are compulsory for any person who will attend the onsite training.
General Overview
Friday, 14 November 2025 11-13 CET
In this webinar, we will focus on how to develop a successful application. The organisation of the proposal application will be presented (excellence, impact, quality and efficiency of the implementation). Moreover, suggestions will be provided for robust proposal development including key steps, and support from specific organisations.
We will then focus on the aspects that are specific to clinical studies and close with an overview of how proposals are evaluated.
Regulatory, ethical, and data framework
Friday, 21 November 2025 11-13 CET
In this webinar, we will focus on the European and National regulatory requirements, ethical requirements and general data protection regulations. We will also discuss the data management plan.
Organisation of the Study
Friday, 28 November 2025 11-13 CET
In this webinar, we will describe how one should organise the clinical study within the proposal framework including elements such as roles and responsibilities, task distribution, study coordination, risk assessment, study management and close out. It will also covers aspects related to patient and public involvement.
Study Budget
Friday, 5 December 2025 11-13 CET
In this session, we will focus on EU format for the budget. The focus will also cover important aspects when preparing a budget and considerations for national specificities.
Webinar Registration
To register you must be situated in a Horizon Europe eligible country. You can register directly for all the webinars or select those that are of interest to you with the single registration link. Applications for onsite will be made available in the following weeks and require presence at the webinars
Register here for online sessions
For any questions or for more information contact media@ecrin.org.
More information on the Onsite training
Please only register at the location of your first choice - you will be offered the option to indicate another preference of location in the dedicated registration form.
05 Feb | Paris, France - Register here
10 Feb | Warsaw, Poland - Register here
13 Feb | Madrid, Spain - Register here
19 Feb | Rome, Italy - Register here
Please note that you are responsible for your own travel and accommodation. As places are limited, please only apply if you are sure to be able to attend the session. Application does not guarantee a spot; if necessary, due to a high number of applications, selection will be made based on the information included in the registration form. Attendance at the webinars (Nov 14- Dec 5) is mandatory. We will confirm the list of accepted attendees for all locations by mid December.
Throughout the course of the day, attendees will have the opportunity to analyse different aspects of the proposal submission procedure. In the morning, we will go through two different use cases. Where possible, the use case will highlight elements of the proposal application process for projects where the host country has had a key role. You will have the opportunity to discuss with some of those involved in the process. In the afternoon, attendees will work together in groups to prepare a proposal (or elements of a proposal) for submission.
Please note that it is compulsory for all attendees of this one day onsite training to join us for the 4 core webinars.


